

Its boiling point is the lowest among all the elements. A the ionic radius of phosphorus Explanation. The atomic radius of Phosphorus atom is 107pm covalent radius. It must be noted atoms lack a well-defined outer boundary. Also some applications for it and some of its compounds which glow in the dark. It must be noted atoms lack a.Ītomic Mass 30973762 Learn more about the atomic mass. Elemental sulfur forms S 8 rings of atoms. Some is used in fireworks safety matches and incendiary weapons. Uranium is a chemical element with atomic number 92 which means there are 92 protons.Ĭhemistry QA Library The atomic radius of sulphur is smaller than phosphorus yet the first ionisation energy of phosphorus is greater than sulphur. All atoms ions have an ionic radius even Phosphorus.
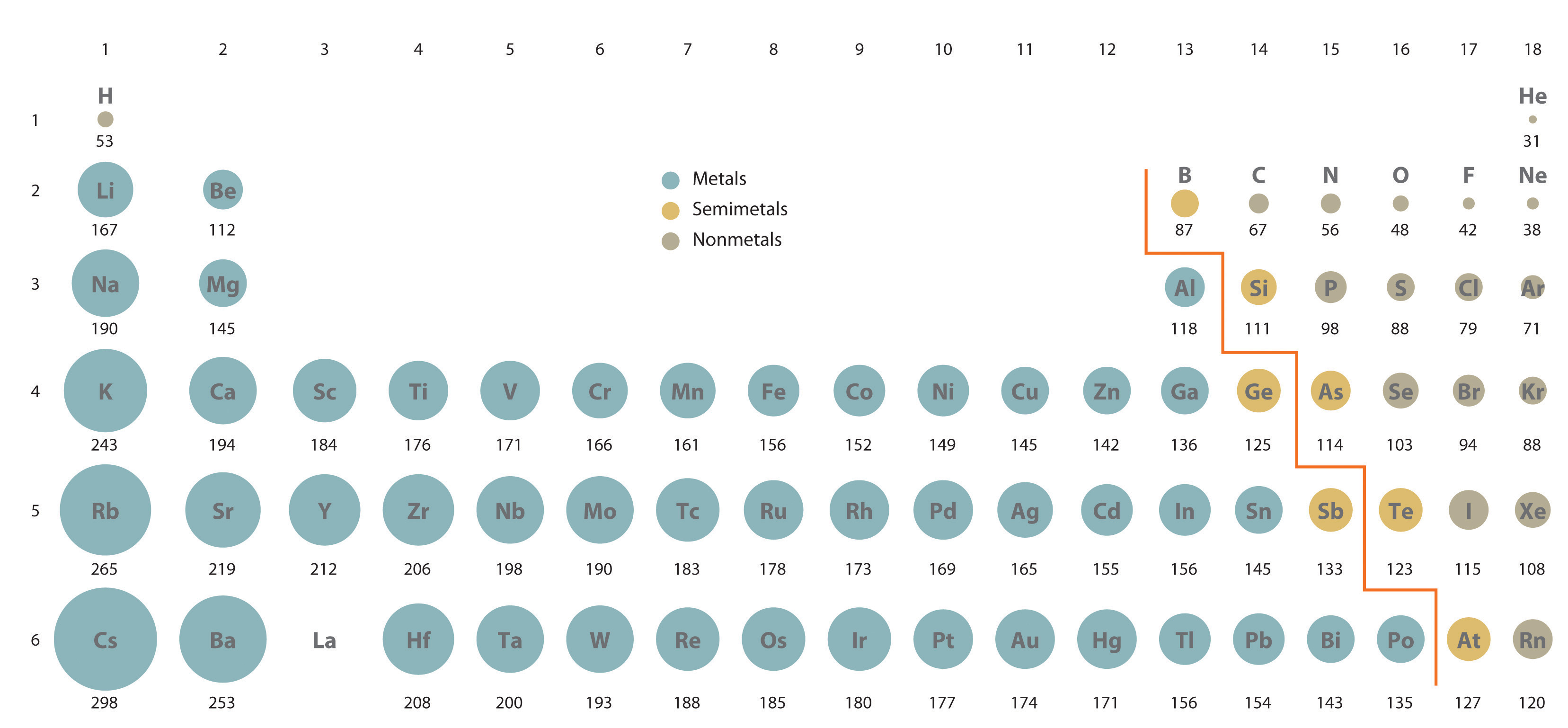
Sources Found most often in phosphate rock. Orbital Radius pm Radius AU Periodicity link. Speaking of Phosphorus let me walk you through some interesting details. MrDotMa 4 weeks ago Chemistry College 10 pts. 119 Zeilen Atomic radius of Europium Eu 233 pm. In the case of Phosphorus the ionic radius is 17 5 Å. Answered Which of the following is larger then the atomic radius of phosphorus Options a.

It is also in the group 15 along with nitrogen and has a molecular weight of 31 g mol-1. The electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. Phosphorus Data Phosphorus Atomic Radius 123 Å State at 20 C solid Uses Used in the production of fertilizers and detergents. Explain why this is so Explain why this is so The atomic radius of sulphur is smaller than phosphorus yet the first ionisation energy of phosphorus. Phosphorus is the 15 th element in the periodic table with the symbol P. Valence shell orbital radii for phosphorus. It is a multivalent atom and can form 3 5 cations. Elemental phosphorus adopts the tetrahedral P 4 arrangement. Which of the following is larger then the atomic radius of phosphorus Options a.ģ question which of the following is larger than the atomic radius of phosphorus p. Phosphorus286 for phosphorus to become stable 288 it gains two electrons thus. The molecules are bigger than phosphorus molecules and thus the van der Waals attractions are stronger leading to a higher. Click here to get an answer to your question which of the following is larger then the atomic radius of phosphorus Options a. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Phosphorus Facts Phosphorus Ionic Radius17 5 Å Discovery Discovered By. Compared to the atomic radius of a carbon atom the atomic radius of a silicon atom is larger because of an increase in. Answers 1 Brynlee Jennings 17 September 1915. Ok so what is the ionic radius of a Phosphorus ion. Phosphorus has several isotopes but P-31 is common with 100 abundance. Ne 3s 2 3p 3.Īll measurements given are in picometers pm. The atomic size of silicon is greater than _. Instead it disrupts the much weaker van der Waals forces between the molecules. Understanding Atomic Radius Trends The 2 Key Principles Melting phosphorus breaks no covalent bonds.Ītomic radius of phosphorus. 7 Zeilen The atomic radius of Phosphorus atom is 107pm covalent radius.


 0 kommentar(er)
0 kommentar(er)
